Abstract
In spite of high rates of complete remission following chimeric antigen receptor (CAR) T cell therapy, the efficacy of this approach is limited by generation of dysfunctional CAR T cells in vivo, conceivably induced by immunosuppressive tumor microenvironment (TME) and excessive antigen exposure. Exhaustion and senescence are two critical dysfunctional states that impose a pivotal hurdle for successful CAR T cell therapies. Recently, modified CAR T cells with an “exhaustion-resistant” phenotype have shown superior antitumor functions and prolonged lifespan. In addition, several studies have indicated the feasibility of senescence delay in CAR T cells. Here, we review the latest reports regarding blockade of CAR T cell exhaustion and senescence with a particular focus on the exhaustion-inducing pathways. Subsequently, we describe what potential these latest insights offer for boosting the potency of adoptive cell transfer (ACT) therapies involving CAR T cells. Furthermore, we discuss how induction of costimulation, cytokine exposure, and TME modulation can impact on CAR T cell efficacy and persistence, while potential safety issues associated with reinvigorated CAR T cells will also be addressed.
Introduction
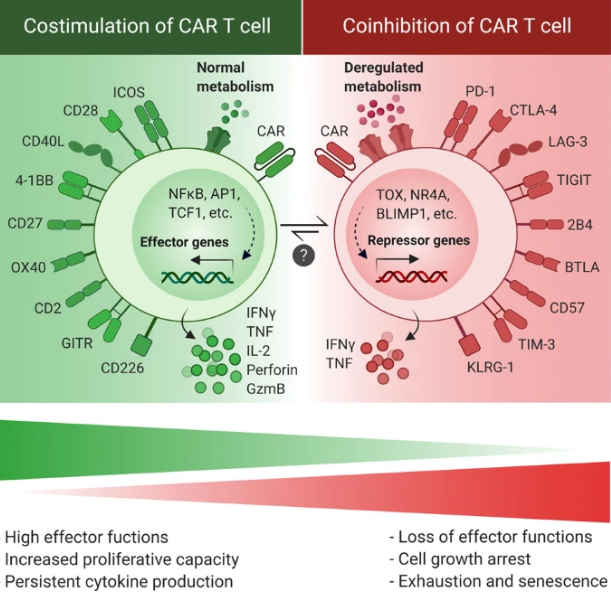
In a recent decade, genetically modified immune cells, particularly chimeric antigen receptor (CAR) T cells, have raised enormous interest in clinical trials [1]. Several generations of CARs have now been developed that are different in the number of intracellular domains or CAR activation mode (Fig. 1). Despite the dramatic clinical benefit of CAR T cell therapy in a broad spectrum of cancer types, a large fraction of patients that achieves remission with CAR T cell therapy displays disease relapse within a few years [2, 3]. Several important explanations of treatment failure in CAR T cell therapies exist, such as tumor antigen escape and inefficient CAR T cell trafficking into the tumor site. However, it is widely thought that limited CAR T cell expansion and persistence in the hostile tumor microenvironment (TME) represent additional key impediments to efficacious CAR T cell responses and durable clinical remission following CAR T cell therapy [4]. The observed reduction in proliferative capacities and persistence of CAR T cells is associated with a generalized dysfunctional phenotype that is hallmarked by impaired proliferative and cytotoxic abilities. Importantly, the root cause for development of this dysfunctional state in CAR T cells is the activation of pathways that promote excessive CAR T cell differentiation, exhaustion, and senescence. Indeed, less-differentiated and less-exhausted CAR T cells have been reported to lead to a better outcome [5]. Central causes for in vivo induction of exhaustion and senescence are persistent stimulation of CAR T cells by high levels of tumor antigens in the face of chronic exposure to a suppressive TME [6]. At the same time, CAR T cell differentiation and exhaustion may be further accelerated by CAR antigen-independent tonic signaling [7].

CAR structure in CAR T cells compose of a single-chain variable fragment (scFv), a hinge and transmembrane (TM) region, costimulatory domain(s) (e.g., OX40, CD28, ICOS, 4-1BB), and a CD3ζ signaling domain. The 1st generation CARs contained only CD3ζ as intracellular domain, while the 2nd or 3rd generations have one or two costimulatory domains linked to CD3ζ, respectively. The 4th generation CARs are known as “TRUCK” CARs. These CARs are structurally similar to the 2nd generation CARs, but with an inducible cytokine expression (e.g., IL-12) through NFAT-responsive promoter.
Given the fact that the efficacy of CAR T cells depends on their capacity to infiltrate the tumor site and directly interact with tumor antigens, in vivo induction of exhaustion and senescence pathways is an unavoidable event in CAR T cell therapies. Indeed, once T cells are activated by the persistent antigen presentation, they subsequently become dysfunctional due to the elevated and sustained expression of inhibitory receptors [8]. Therefore, it is more attractive to focus on approaches to prevent intrinsic dysfunctional pathways in CAR T cells (e.g., inhibitory receptors signaling) and generate “exhaustion-resistant” cells, rather than aiming to modulate their exposure to tumor antigens in the TME. Through this strategy, the “exhaustion-resistant” CAR T cells might maintain their effector functions even during a prolonged exposure to their cognate antigen. In concordance with this, modified CAR T cells with disrupted pathways inducing exhaustion or senescence have shown a significantly higher persistence and antitumor activity, providing a promising outlook for reversal or delay of CAR T cell exhaustion and senescence as a way to harness the full potential of this highly effective treatment modality [9,10,11].
Concept of exhaustion and senescence
Although exhausted T cells display some phenotypic markers that are typically associated with effector and memory states [12], they show phenotypically and functionally different properties from both effector and memory subsets [13]. Since exhaustion and senescence share several overlapping characteristics such as defective effector functions, impaired proliferation, and cell cycle arrest, they might be used interchangeably. However, there are certain characteristics that can be used to distinguish these states from each other, including cytokine secretion signatures, and expression of cell surface receptors and transcription factors [14].
Read more at: https://www.nature.com/articles/s41388-020-01501-x#cancer

Comments are closed